Sudden deflation of the tissue expander used in two-stage breast reconstruction surgery: two cases of this rare complication
Article information
Abstract
For breast cancer patients, the goal after mastectomy is to reconstruct a cosmetically beautiful, natural-appearing breast that is symmetrical to the contralateral breast. Breast reconstruction using a tissue expander is one of the most commonly employed methods. This two-stage method involves initially placing a tissue expander, followed by its exchange for a permanent implant. It is recognized as safe and effective, and demand for this procedure is gradually increasing. However, we encountered two unusual instances of sudden deflation of tissue expanders used in two-stage breast reconstruction surgery. Both expanders were placed using a dual-plane technique beneath an acellular dermal matrix. One patient had undergone radiation therapy, while the other had not. Upon removal of the tissue expanders, they were examined for physical rupture, but no abnormalities were detected. Postoperative malfunction of tissue expanders or implants is relatively uncommon, and there have been no reports of delayed leakage without rupture of the expander. In this study, we present a rare complication involving tissue expanders and discuss potential causes and solutions.
INTRODUCTION
For breast cancer patients, the goal after mastectomy is to reconstruct a cosmetically beautiful, natural-appearing breast that is symmetrical to the contralateral breast. There are various methods and timing options for breast reconstruction, and the outcomes can vary based on the patient’s personal preferences, physical circumstances, and oncological status. In recent years, immediate breast reconstruction has become the most commonly performed surgery for breast cancer patients. If a skin-sparing mastectomy is performed, or if the blood circulation in the skin flap is anticipated to be suboptimal, a tissue expander may be utilized. Breast reconstruction using a tissue expander is one of the most common reconstructive methods, with over 80,000 such procedures carried out in the United States in 2020 [1]. Recognized for its safety and effectiveness, the two-stage reconstruction method, which involves an initial placement of a tissue expander followed by its replacement with a permanent implant, is increasingly popular [2,3]. With the growing demand for this method, research into postoperative complications and associated factors has also increased. Mastectomy, skin flap necrosis, and breast infection are the most common complications associated with reconstruction using a tissue expander [4]. However, there are limited studies and reports on complications arising from sudden or gradual contraction of the tissue expander. In this paper, we report two rare cases of sudden deflation of a tissue expander used in two-stage breast reconstruction surgery.
CASE REPORT
Case 1
A 46-year-old woman diagnosed with invasive ductal carcinoma of the right breast underwent a skin-sparing mastectomy with axillary lymph node dissection. Immediate breast reconstruction was performed using a 550-cc tissue expander (Mentor Worldwide LLC), which was initially inflated with 250 cc of saline at the time of surgery. The tissue expander was placed in a dual-plane position, supported by an 18×8 cm acellular dermal matrix (ADM). At her first outpatient clinic follow-up at 2 months post-operation, an additional 20 cc of saline was added to the expander. Further inflations were put on hold during her chemotherapy regimen, which consisted of four cycles of doxorubicin and cyclophosphamide and four cycles of docetaxel and trastuzumab, as well as during her 28 sessions of radiation therapy. After a sufficient healing period for the breast tissue, saline inflations resumed at 2-month intervals, with 50 cc added per session, for a total of six times. During these subsequent inflations, it was noted that although the tissue expander would initially expand, the expansion was not sustained over time. The patient had no history of trauma. Suspecting spontaneous leakage from the tissue expander, a second surgery was scheduled earlier than anticipated, despite the skin tissue not having expanded adequately. During this procedure, the tissue expander was removed and replaced with a 340-cc cohesive gel implant (MemoryGel Smooth Round; Mentor Corp.). Due to the larger size of the contralateral breast, symmetrical reconstruction was not achievable, but the surgery was completed successfully without further complications (Fig. 1). After the removal of the tissue expander, it was examined for any physical rupture, but no abnormalities were found. In the operating room, immediately after its removal, saline was injected into the expander, yet no leakage was detected. Furthermore, the saline solution remained intact even when the expander was left in that state for three months (Fig. 2A).

Photographs of the case 1. (A) Photograph of the patient taken prior to surgery. (B) After skin-sparing mastectomy, immediate reconstruction was performed with a 550-cc tissue expander and an 18×8 cm acellular dermal matrix. During the operation, the expander was inflated with 250 cc of saline.
Case 2
A 47-year-old woman underwent a nipple-sparing mastectomy with sentinel lymph node biopsy due to ductal carcinoma in situ in the right breast. Immediate breast reconstruction involved the insertion of a 450-cc tissue expander, which was initially injected with 250 cc of saline, using a dual-plane technique with an 18×8 cm ADM (Fig. 3). Following discharge, the patient began taking 20 mg of tamoxifen daily as an outpatient. Approximately 1 year after surgery, the patient observed a sudden reduction in breast volume without any preceding trauma and promptly sought medical attention. Since the patient had sufficient skin to proceed with the final surgery, plans were made to operate without delay. The tissue expander was extracted, and a 270-cc cohesive gel implant was inserted. In alignment with the patient’s preference, a 130-cc cohesive gel implant (MemoryGel Smooth Round; Mentor Corp.) was used for augmentation mammoplasty on the left breast concurrently (Fig. 4). Postoperatively, the symmetry of both breasts was satisfactory, and no complications occurred. Additionally, the integrity of the tissue expander was assessed for physical rupture, but no defects were detected. Upon its removal in the operating room, the expander was tested by injecting saline, yet no leakage was found. Furthermore, the saline solution remained intact even after being left undisturbed for 3 months (Fig. 2B).
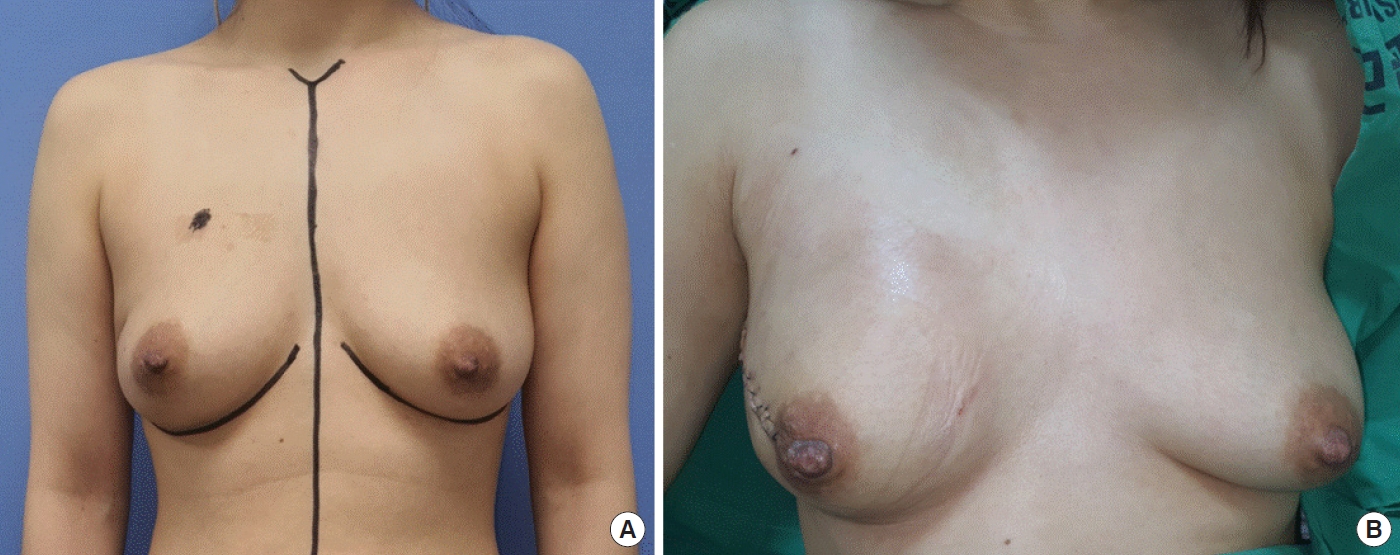
Photographs of the case 2: preoperative status and immediate reconstruction. (A) Photograph of the patient taken prior to surgery. (B) After nipple-sparing mastectomy, immediate reconstruction was performed with a 450-cc tissue expander and an 18×8 cm acellular dermal matrix. During the operation, the expander was inflated with 250 cc of saline.

Photographs of the case 2: expander deflation and the subsequent surgical intervention. (A) Twelve months after surgery, the patient visited the hospital because the tissue expander suddenly deflated without any traumatic event. (B) The final surgery was promptly planned, and the tissue expander was replaced with a 270-cc silicone implant to prevent further skin contraction. At the patient's request, breast augmentation surgery was also performed on the contralateral breast using a 130-cc silicone implant. The patient was satisfied with the surgical outcomes for both breasts.
DISCUSSION
Breast cancer is currently one of the most prevalent cancers among women. The loss of a breast to cancer can be a deeply distressing experience, significantly affecting a woman’s emotional well-being and quality of life. For those who have undergone a mastectomy, breast reconstruction provides benefits that are both aesthetic and psychosocial [5,6]. While there are many techniques for breast reconstruction, nearly half of all reconstructions are performed using the tissue expander/implant method. This approach is favored because it is safe, cost effective, and reliable, and it can be applied to women with a broad range of comorbidities [7,8].
Numerous studies have demonstrated the safety of tissue expanders in breast reconstruction [9]. Tissue expanders remain one of the most commonly employed surgical techniques, alongside silicone implants and autologous tissue reconstruction. Compared to autologous tissue reconstruction, tissue expander/implant-based reconstruction offers several advantages, including shorter operative times, quicker recovery, and the absence of donor site morbidity.
Nevertheless, there are complications that can arise with reconstruction using tissue expanders. Complications following tissue expander/implant-based reconstruction are categorized into early and late complications. The most common early complications are infection, hematoma, seroma, and necrosis of the nipple-areolar complex or skin flap. Late complications may include capsular contracture, as well as exposure or loss of the expander or implant. However, with appropriate treatment, almost every complication can be completely resolved.
Hematoma formation, most commonly occurring on the first or second day after surgery, is observed in 0% to 5.8% of patients [10]. Effective drainage allows for the timely detection and management of bleeding. However, if blood clots obstruct the drainage, blood accumulation in the pocket becomes likely, necessitating prompt reoperation. Hematomas, regardless of size, may precipitate capsular contracture and thus require immediate evacuation.
Breast skin flap necrosis can occur in up to 21% of patients following tissue expander insertion [10]. After a mastectomy, patients may experience either partial or total necrosis of the breast skin flap, which typically begins at the incision line. If flap necrosis is suspected, it is necessary to apply topical antibiotics and provide local wound care. In cases where the necrotic area is localized, wound revision may be required to address the skin breakdown. If the area of skin necrosis is extensive, deflating the tissue expander or removing the implant might be the only viable option. It is important to note that reinsertion of a tissue expander or implant should not be scheduled until at least 36 months after surgery.
The risk of complications with tissue expanders or implants is minimal in the early stages of surgery. Ensuring the correct placement of tissue expanders and verifying the functionality of the port during the procedure are critical steps to prevent complications. However, late complications can occur at any time. The rates of delayed expander/implant deflation and rupture increase with the age of the implants, reaching 15% between 3 to 10 years post-implantation [11]. Diagnosing these issues can be challenging; not all cases result in changes to breast size and shape, and mammograms do not consistently detect implant ruptures. Magnetic resonance imaging is the most reliable method for identifying implant ruptures, with a sensitivity of 86.7% and a specificity of 88.5% [11]. In cases where rupture of the tissue expander or implant is strongly suspected, surgical exploration and implant replacement are warranted.
Research on tissue expander rupture is relatively scarce; therefore, we analyzed the results of existing studies on implant rupture. Several studies have investigated the mechanisms underlying implant rupture. Suspected causes of implant rupture include damage from surgical instruments, shell swelling, fold flaws, or direct trauma to the implant during closed capsulotomy [12]. Handel et al. [13] reviewed data from Mentor (Mentor Corp.) and Allergan (Allergan Corp.) regarding the causes of rupture. They found that 50% to 64% of ruptured implants were reportedly damaged by surgical instruments. This was followed by cases of unidentified openings or tears without a clear cause, with no evidence of sharp instrument damage or wear due to prolonged friction, accounting for 35% to 37% of ruptures.
According to an extensive search of previous studies, the most common reasons for the removal of tissue expanders have been identified as infection or exposure of the device. Instances of delayed leakage without rupture of the tissue expander have not been documented. However, in our case series, two patients experienced either gradual or sudden deflation of their tissue expanders. To determine the cause, we examined the tissue expander for physical rupture immediately upon removal, yet no abnormalities were detected. For a more thorough investigation, we injected saline into the removed tissue expander and observed it for leaks, but none were found. Subsequently, the tissue expander was monitored for 3 months to detect any changes over time; the saline level remained unchanged.
Regarding the causes of these cases, we presume that the first case occurred due to increased pressure on the tissue expander as a result of skin contracture following radiation therapy. In the second case, despite the absence of radiation therapy, the patient desired an enlargement of the contralateral breast. Consequently, the breast tissue was expanded beyond its original size, which we speculate may have led to excessive pressure on the expander, resulting in its sudden deflation. Existing research has investigated the amount of pressure exerted on a tissue expander within the human body [14]. Another study has indicated a higher incidence of complications with tissue expanders placed in the lower extremities, particularly in younger patients [15]. The frequent complications associated with tissue expanders in the lower extremities are believed to be related to pressure. However, to date, there has been no research specifically addressing complications caused by pressure.
Our study has several limitations. First, it included only two cases. Additionally, the definitive cause of the observed cases has not been established. In the operating room, immediately after the removal of the tissue expander, it was filled to maximum capacity with saline and monitored for over a month. During this period, without any external pressure applied, there was no leakage, and the inflation remained stable. This observation prompted us to report these cases. However, we have not conducted further research to examine the outcomes when external pressure is exerted. Future studies are planned to investigate the resilience of tissue expanders to external pressure and to identify the threshold at which contraction occurs. While most surgeons focus exclusively on complications such as hematoma, seroma, infection, and flap necrosis, our findings suggest that the pressure applied to the expander pocket is also a critical factor. Therefore, the techniques for overfilling must be carefully observed.
In conclusion, when performing breast reconstruction with a tissue expander, it is essential to ensure that the pocket for the expander is adequately secured to minimize physical pressure. Additionally, if leakage of the tissue expander is suspected, it is more advantageous to promptly plan the subsequent stage of surgery rather than persisting with inflation attempts. We hope that the findings of this study will contribute to improved outcomes in future breast reconstructions utilizing tissue expanders.
Notes
No potential conflict of interest relevant to this article was reported.
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki. The study was approved for exemption by the Institutional Review Board of Hanyang University Guri Hospital.
Patient consent
Written informed consent from the patient was obtained from the patients for the use of their photographs.

