|
 |
- Search
| Arch Aesthetic Plast Surg > Volume 30(1); 2024 > Article |
|
Abstract
Gigantomastia is a rare condition characterized by excessive hypertrophy of the connective tissue of the breast, which can cause physical and emotional distress. Surgical intervention is crucial for improving patients’ quality of life; however, it is challenging to balance minimizing the risk of recurrence and maximizing favorable aesthetic outcomes. This study documents the successful management of three rare cases of bilateral gigantomastia resulting from benign tumors. These cases included rapidly growing bilateral diffuse tumorous pseudoangiomatous stromal hyperplasia (PASH) and bilateral juvenile giant fibroadenoma, with no long-term recurrence observed. We discuss the diagnostic challenges and management considerations for gigantomastia, with a focus on reviewing PASH and its differential diagnosis. The findings offer valuable insights into the successful management of diverse gigantomastia cases caused by benign tumors, potentially aiding clinicians in making more informed decisions regarding optimal patient care.
Gigantomastia is a rare condition characterized by excessive hypertrophy of the connective tissue of the breast. While the exact criteria vary across studies, gigantomastia is typically defined as breast tissue comprising more than 3% of total body weight, excessive breast growth exceeding 1.5 kg per breast, or overly large breasts requiring reduction by 1–2 kg [1]. This condition can cause both physical and emotional distress, making surgical intervention crucial for improving patient quality of life. Surgical treatments include reduction mammoplasty or mastectomy with reconstruction. Reduction mammoplasty can yield favorable aesthetic and symptomatic outcomes, whereas mastectomy is sometimes considered overly aggressive. However, reduction mammoplasty carries a risk of recurrence, with a reported recurrence risk ratio of greater than 5.0 compared to mastectomy [2]. Therefore, performing reduction presents technical challenges. These include minimizing the risk for recurrence by removing sufficient tissue while maintaining vascular support, as well as establishing an aesthetically acceptable breast mound despite the severe degree of ptosis.
Certain tumors infrequently result in gigantomastia. This article presents three cases of bilateral gigantomastia induced by benign tumors, including the extremely rare condition of bilateral diffuse tumorous pseudoangiomatous stromal hyperplasia (PASH), and includes long-term follow-up data. To our knowledge, no previous long-term follow-up studies have addressed the successful management of bilateral diffuse tumorous PASH. In this study, medical records were retrospectively reviewed in accordance with institutional guidelines.
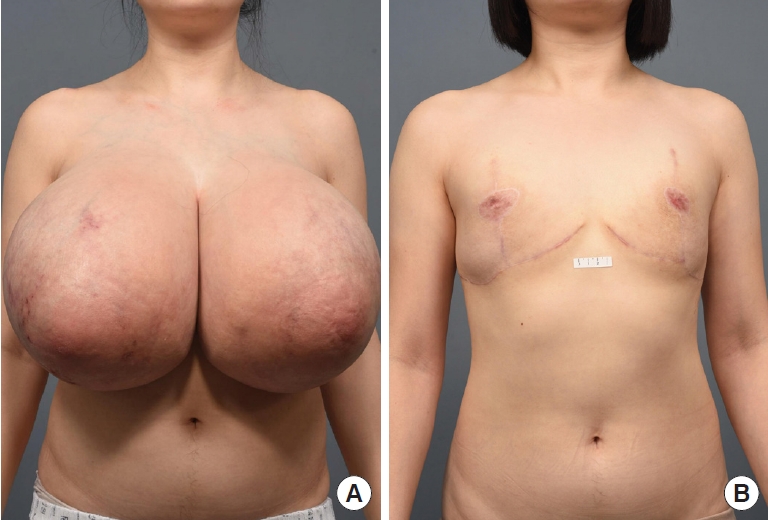
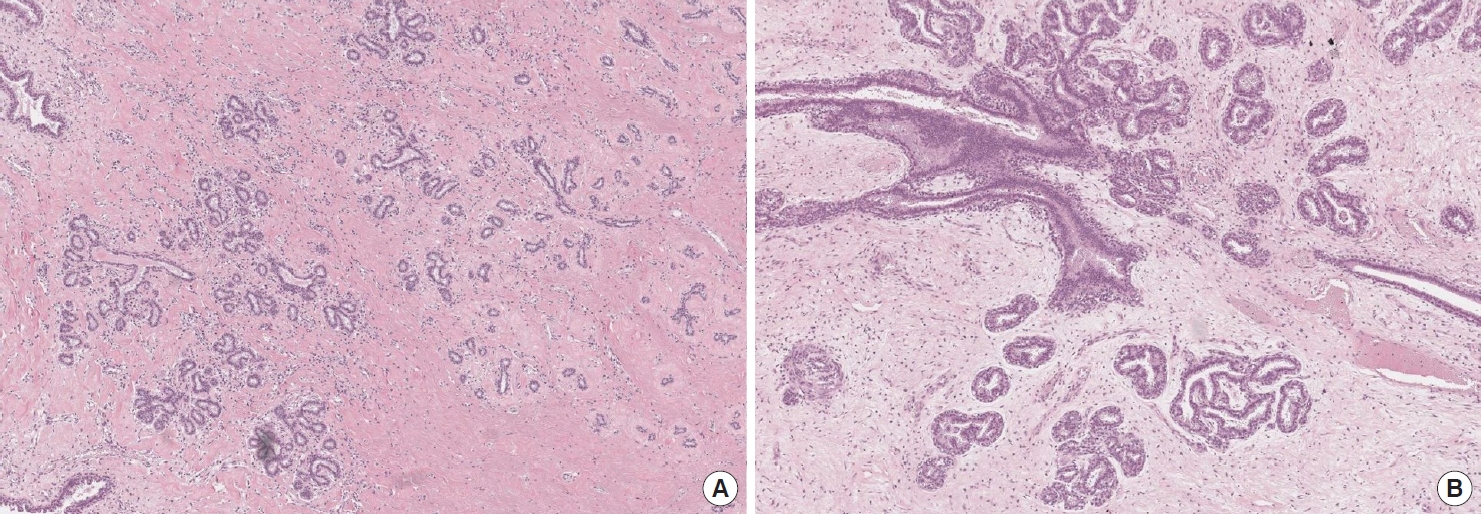
A 27-year-old woman presented with a 2-year history of progressive bilateral breast enlargement and bilateral galactorrhea (Fig. 1A). She was otherwise healthy, with no history of taking hormonal or immunosuppressive medications, and had a body mass index of 26.5 kg/m2. Physical examination revealed bilateral symmetrical gigantomastia without palpable nodules. Breast mammography and sonography indicated diffuse ductal dilatation with no definite lesions (Fig. 2A and B). The patient exhibited a normal hormonal profile, including prolactin levels. However, she reported experiencing physical and emotional distress, leading her to undergo bilateral reduction mammoplasty using the inverted-T design with free nipple grafting. The entire inferior pole of the breast was removed without a pedicle, and the upper medial and lateral skin flaps were used to form the breast mound. The right and left specimens measured 30×27×9 cm (4.6 kg) and 29×28×8 cm (4.5 kg), respectively. Serial sectioning of both breasts revealed that the entire breast parenchyma had been replaced by fibrous, lumpy, and mucoid tissues (Fig. 2C and D). Microscopic examination showed stromal hyperplasia and spindle cells lining the empty slit-like spaces. Immunohistochemical staining revealed stromal cells positive for ERG and spindle cells positive for CD34 (Fig. 3). The final diagnosis was bilateral diffuse PASH. The patient was discharged on postoperative day 4 without acute complications. At 2.3 years postoperatively, no recurrence was observed; additionally, the nipple grafts were well taken, and the breast mound was well maintained (Fig. 1B).
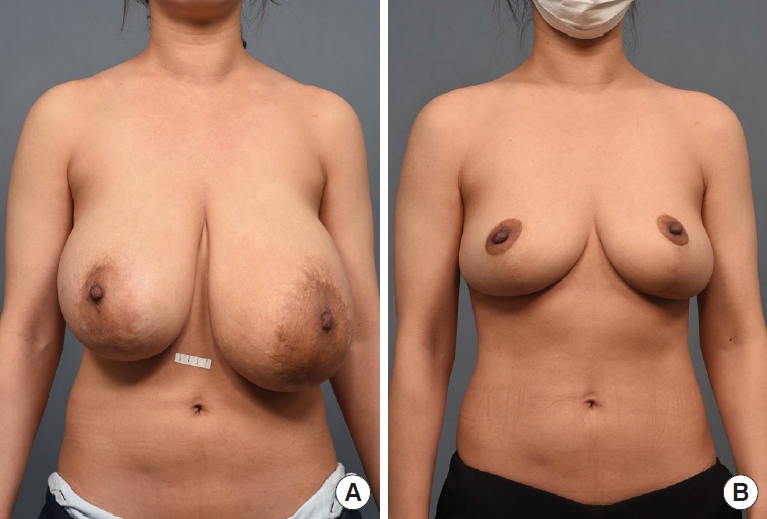
An 18-year-old female patient presented with rapid bilateral asymmetric breast enlargement that began around the time of her first menstrual period, at 14 years of age (Fig. 4). She had been diagnosed with bilateral juvenile fibroadenoma based on fine-needle aspiration and had been treated with gonadotropin-releasing hormone agonist injections for 2 years. Breast enlargement had continued for 1 year after hormonal therapy was discontinued. The patient reported chronic pain in her lower back, neck, and shoulders, associated with lumbar scoliosis. This pain was causing her substantial stress during physical activities at school. Consequently, the patient opted for surgical intervention. Palpation revealed bilateral firm, non-tender, well-defined masses, and the larger left breast exhibited hyperpigmented skin changes. Sonography indicated huge circumscribed heterogeneous echoic masses with septations involving the entirety of both breasts (Fig. 5A and B). No hormonal abnormalities were detected during preoperative consultation at the pediatric endocrinology clinic. Bilateral reduction mammoplasty was performed using the inverted-T design. For the right breast, the nipple was repositioned based on a superomedial pedicle, while for the left breast, a free nipple graft was performed. The right and left specimens measured 11.5×9.7×5.5 cm (0.7 kg) and 19.9×16.7× 12.0 cm (1.8 kg), respectively. Macroscopic examination of the bilateral specimens revealed bilateral solid, encapsulated, well-defined masses with multiple septations, which occupied almost the entirety of the breast specimens (Fig. 5C and D). A right fibrous mass (11.0×10.0×5.5 cm) and a left myxoid mass (18.0×14.0×12.0 cm) were consistent with juvenile fibroadenoma, accompanied by stromal and epithelial hyperplasia without necrosis (Fig. 6). The patient was discharged on the second postoperative day without acute complications. At 5.5 years postoperatively, no recurrence was observed; furthermore, the left nipple graft was well taken, and the bilateral breast mounds were well maintained.
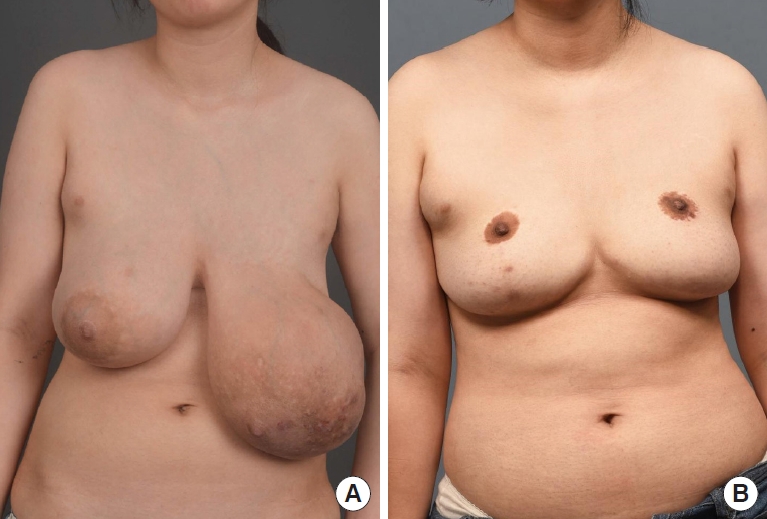
A previously healthy 40-year-old woman, with a body mass index of 22.2 kg/m2, presented with a 10-year history of progressive bilateral asymmetric breast enlargement without other symptoms such as nipple discharge or mastalgia (Fig. 7A). The left breast was larger than the right, and ill-defined masses were palpated bilaterally. Sonography and mammography revealed multiple masses of varying size and density in both breasts. Bilateral reduction mammoplasty was performed using the inverted-T design based on a superomedial pedicle. The right and left specimens measured 16×9×8 cm (1.0 kg) and 18×16.5×9 cm (1.6 kg), respectively. Serial sectioning of specimens from both breasts revealed multiple poorly circumscribed masses dispersed throughout the parenchyma. Microscopic examination of the right specimen revealed numerous multifocal fibroadenomas, measuring up to 2.5×1.5×1.5 cm. Furthermore, an ill-defined mass was identified, measuring 2.7×1.7×1.5 cm; this mass was composed of enlarged and distorted lobules with stromal fibrosis, consistent with sclerosing adenosis. Microscopic examination of the left breast specimen revealed multiple fibroadenomas of up to 2.0×1.5×0.5 cm, along with overall fibroadenomatoid changes and ductal ectasia within the parenchyma. The patient was discharged on the second postoperative day without acute complications. At 1.5 years after surgery, no recurrence was observed, and the aesthetic appearance of the breast mound was well preserved (Fig. 7B).
Gigantomastia is a rare disorder considered to be associated with hormonal changes. As such, it can manifest during puberty or pregnancy, in the presence of autoimmune diseases, or as a result of drug use. Certain tumors can also induce gigantomastia. However, these cases may be preoperatively classified as idiopathic gigantomastia, as preoperative imaging examinations sometimes reveal no definitive lesions [1,2].
While substantial breast size can lead to meaningful psychological and physical distress, securing national health insurance coverage for breast reduction surgery in South Korea is challenging. Unless surgery is required due to tumor-related causes, as in the cases mentioned above, insurance approval typically necessitates documented proof of chronic skin eczema, neck and shoulder deformities, or other impairments that affect daily life due to the presence of gigantomastia [3]. In the United States, insurance providers employ assessment tools, such as the Schnur scale, to determine the minimum breast resection weights that justify the medical need for reduction surgery. Typically, insurance companies will cover this procedure if the amount of tissue to be removed exceeds the 78th percentile based on the individual’s body surface area or weighs more than 500 g [4].
This article presents several uncommon cases of bilateral gigantomastia characterized by conditions such as diffuse PASH, juvenile giant fibroadenoma, and multiple fibroadenomas concurrent with sclerosing adenosis. To our knowledge, this is the first case series to document a rapidly growing bilateral diffuse giant PASH that exhibited no long-term recurrence following surgical excision.
PASH is a rare benign tumor that typically presents as a localized nodular breast mass with slow progression. Incidental PASH changes, including at least one microscopic focus, have been reported in 23% of cases, while nodular PASH has an incidence of 0.4% in breast biopsies [5]. Rapidly growing bilateral diffuse PASH is extremely rare, and even the overall incidence of diffuse PASH has not yet been established. Diffuse PASH can present as multiple nodular lesions or as extensive lesions involving the parenchyma without obvious masses. The present case aligns with the latter presentation. The patient demographic for PASH is broad, encompassing individuals from teenagers to postmenopausal women [6].
The term “pseudoangiomatous” originates from the histological features characteristic of PASH, which resemble vascular structures. This condition presents as a dense, collagenous proliferation of the mammary stroma, with interconnecting capillary-like spaces lined by slender spindle cells. The nuclei of the spindle cells are attenuated, show no signs of atypia, and do not exhibit mitotic activity. Immunohistochemistry reveals that the spindle cells lining these spaces exhibit strong immunoreactivity for vimentin and CD34, but do not react to CD31 and factor VIII. The pathophysiology of this condition remains undefined; however, some studies propose a progesterone-primed neoplastic process in myofibroblasts. Immunohistochemical staining is usually positive for progesterone and estrogen receptors [7]. This finding is supported by the fact that over half of postmenopausal women with PASH tumors have undergone hormone replacement therapy [6]. In contrast, our patient with PASH did not exhibit any hormonal abnormalities or have a history of hormonal therapy.
The diagnostic workups for this condition include mammography and sonography. Nodular PASH is characterized by solid hypoechoic masses with circumscribed and cystic changes on sonography, as well as by round, ovoid, non-calcified masses on mammography. In contrast, diffuse PASH presents as a diffuse increase in density involving the parenchyma on mammography, and as multiple dispersed circumscribed or obscured heterogeneous lesions with minimal vascularity on sonography. However, no defining imaging features have been established, and only 31% of cases involving palpable PASH can be distinguished on mammograms [8,9]. Regarding management, small lesions that progress slowly can be monitored, and medications such as selective estrogen receptor modulators may be beneficial. In severe cases, surgical treatment is considered, although the recurrence rate after excision is reportedly as high as 15% to 22%. No distant metastases or deaths associated with PASH have been documented, nor has the condition been linked with an increased risk of breast cancer [10].
PASH can mimic fibroadenoma, benign phyllodes tumors, or low-grade angiosarcoma and may coexist with the other breast lesions previously mentioned. Fibroadenoma is the most common benign tumor of the female breast. Unlike PASH, it generally grows slowly and unilaterally. This tumor is characterized by a well-circumscribed, non-encapsulated lesion with a combination of epithelial and stromal elements. Juvenile fibroadenoma, a rare variant, accounts for 0.5%–2% of all fibroadenomas. It is encountered in the adolescent population as a rapidly growing, sizable mass (>10 cm) [11]. This variant presents as an encapsulated and intraductal hyperplasia of the epithelial components. On ultrasound, it appears as a well-defined, oval, hypoechoic or isoechoic mass with posterior acoustic enhancement and hypervascularity. The risk of malignant transformation of juvenile fibroadenomas is reported to be less than 0.3%, which is slightly higher than that of simple fibroadenomas. Symptomatic or large tumors necessitate surgical treatment. During surgery, it is crucial to preserve as much as possible of the surrounding normally developed breast parenchyma and nipple-areolar complex. The remaining breast tissue gradually fills the empty space after tumor excision, eliminating the need for further reconstructive procedures. However, large juvenile fibroadenoma lesions can compress the surrounding healthy breast tissue, complicating accurate assessment of the unaffected parenchyma [12,13].
Like juvenile fibroadenomas, benign phyllodes tumors are well-defined and encapsulated, but their growth rate can be either slow or rapid. These tumors display a distinctive fibroepithelial leaf-like growth pattern when examined histologically. On ultrasonography, they appear as inhomogeneous solid masses, featuring cleft-like cystic spaces and posterior acoustic enhancement. Unlike juvenile fibroadenomas, benign phyllodes tumors necessitate wide excision, including a rim of normal tissue, both to prevent recurrence and to ascertain whether the tumor is benign, borderline, or malignant [14].
Low-grade angiosarcoma may also be misdiagnosed as PASH, and only histological examination can confirm the diagnosis. This condition typically presents as a rapidly growing, palpable mass, and early detection is vital due to its poor prognosis. The 5-year disease-free survival rate for low-grade tumors is reported to be between 70% and 76%. On sonograms, low-grade angiosarcoma can appear as a heterogeneous lesion with both hyperechoic and hypoechoic characteristics. On mammography, it may present as a large, dense, homogeneous, sharply defined, non-calcified mass [15].
In conclusion, a variety of tumors can lead to gigantomastia, and diagnosis is often challenging based on clinical presentation and radiological examination alone. To manage this condition, underlying medical conditions should be identified, including endocrine or oncological issues. This article presents the successful management of several cases of bilateral gigantomastia, providing clinicians with insights that may help them consider a range of differential diagnoses to develop more targeted management strategies.
Notes
Ethical approval
The study was approved by the Institutional Review Board of Seoul National University Hospital (IRB No. 2302-133-1408).
Patient consent
The patients provided written informed consent for the publication and use of their images.
Fig. 2.
(A, B) Preoperative breast sonography revealing other than diffuse ductal dilatation. (C, D) Excised left breast specimen and serial sectioning of the specimen.
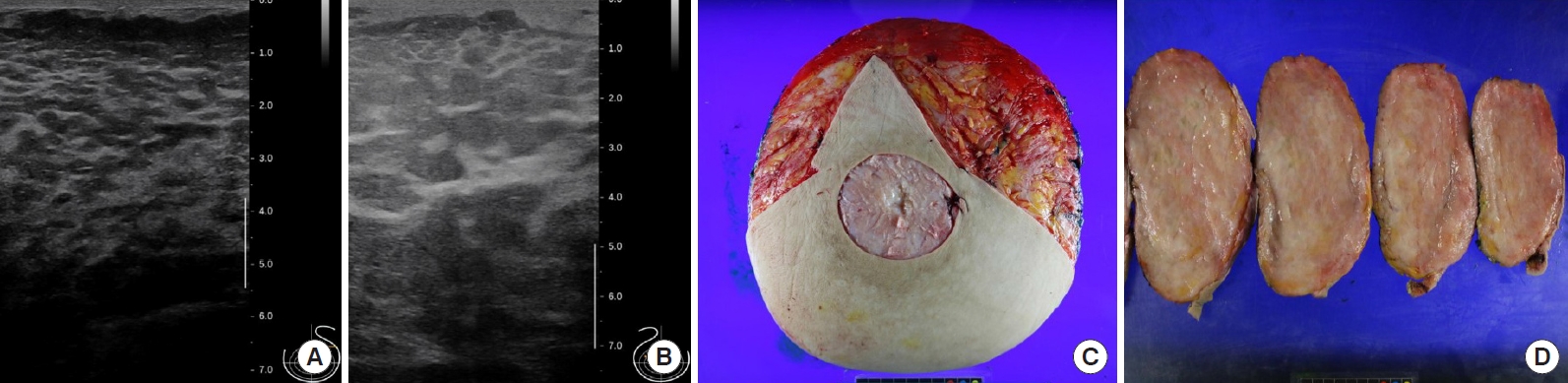
Fig. 3.
Postoperative histopathological slides of the specimen (×200). (A, B) Hematoxylin and eosin staining. (C) Immunohistochemical staining for CD34. (D) ERG staining for CD34.
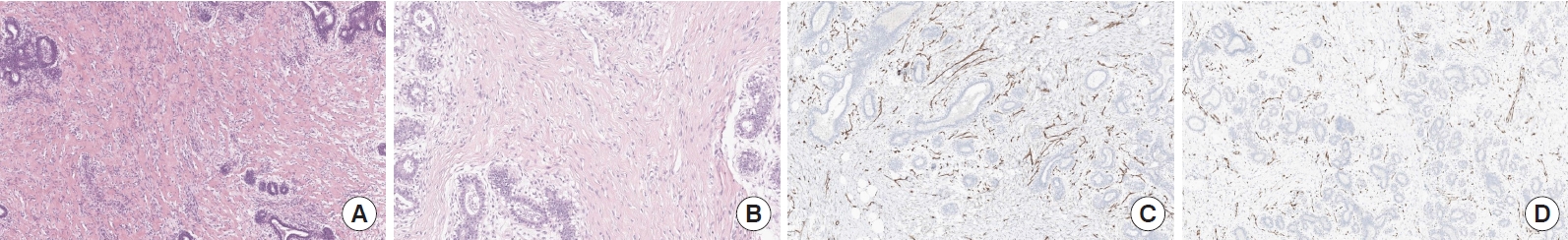
Fig. 5.
(A, B) Preoperative breast sonography revealing huge heterogeneous echoic masses. (C, D) Excised left breast specimen and serial sectioning of the specimen.

REFERENCES
1. Sornlertlumvanich M, Rohitopakarn P, Samphao S, et al. Repeated recurrence of bilateral gigantomastia after subcutaneous mastectomy caused by tumoral pseudoangiomatous stromal hyperplasia: a case report and review of literature. BJR Case Rep 2022;9:20220074.



2. Hoppe IC, Patel PP, Singer-Granick CJ, et al. Virginal mammary hypertrophy: a meta-analysis and treatment algorithm. Plast Reconstr Surg 2011;127:2224-31.


3. Health Insurance Review and Assessment Service. Health Insurance Medical Care Costs (February 2023 edition). Health Insurance Review and Assessment Service; 2023.
4. Yan M, Bustos SS, Kuruoglu D, et al. Breast resection weight prediction and insurance reimbursement in reduction mammaplasty: which scale is reliable? Plast Reconstr Surg 2022;150:723e-730e.


5. Yilmaz E, Gungoren FZ, Yilmaz A, et al. Pseudoangiomatous stromal hyperplasia of the breast: mammosonography and elastography findings with a histopathological correlation. J Breast Health 2015;11:148-51.



6. Testori A, Alloisio M, Errico V, et al. Pseudoangiomatous stromal hyperplasia: a benign and rare tumor of the breast in an adolescent: a case report. J Med Case Rep 2017;11:284.



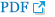
7. Virk RK, Khan A. Pseudoangiomatous stromal hyperplasia: an overview. Arch Pathol Lab Med 2010;134:1070-4.


8. Ryu EM, Whang IY, Chang ED. Rapidly growing bilateral pseudoangiomatous stromal hyperplasia of the breast. Korean J Radiol 2010;11:355-8.



9. Hargaden GC, Yeh ED, Georgian-Smith D, et al. Analysis of the mammographic and sonographic features of pseudoangiomatous stromal hyperplasia. AJR Am J Roentgenol 2008;191:359-63.


10. Jaunoo SS, Thrush S, Dunn P. Pseudoangiomatous stromal hyperplasia (PASH): a brief review. Int J Surg 2011;9:20-2.


11. Salati SA. Breast fibroadenomas: a review in the light of current literature. Pol Przegl Chir 2020;93:40-8.


12. Sosin M, Pulcrano M, Feldman ED, et al. Giant juvenile fibroadenoma: a systematic review with diagnostic and treatment recommendations. Gland Surg 2015;4:312-21.